Imagine a battlefield after a fierce war. The enemy army has been decimated, and the victory banners are raised.
But hidden in the shadows, a handful of rogue soldiers linger and are plotting a comeback. In the world of cancer, this is the essence of minimal residual disease (MRD), in which elusive cancer cells persist in the body after treatment. In many cases, there are still too few cancer cells to detect with conventional methods, but they may be potent enough to spark relapse. Also known as molecular residual disease in some circles, MRD represents the microscopic remnants of malignancy that can turn a hard-won remission into a recurring nightmare.
For decades, harnessing the clinical value of MRD was like chasing a pipe dream. Technological limitations plagued early detection efforts. Traditional methods, such as flow cytometry and polymerase chain reaction, struggled with sensitivity, often failing to spot cancer cells at levels below 1 in 10,000. Cancer heterogeneity and clonal evolution added layers of complexity, in which cancer subclones could evade detection, leading to false negatives. In solid tumors, the challenge was even steeper; accessing tissue samples was invasive, and blood-based approaches lacked the precision to distinguish tumor DNA from normal circulating fragments. These barriers meant MRD testing was more academic curiosity than a clinical tool, leaving patients and clinicians in the dark about true remission status.
The Dawn of the Burgeoning MRD Industry
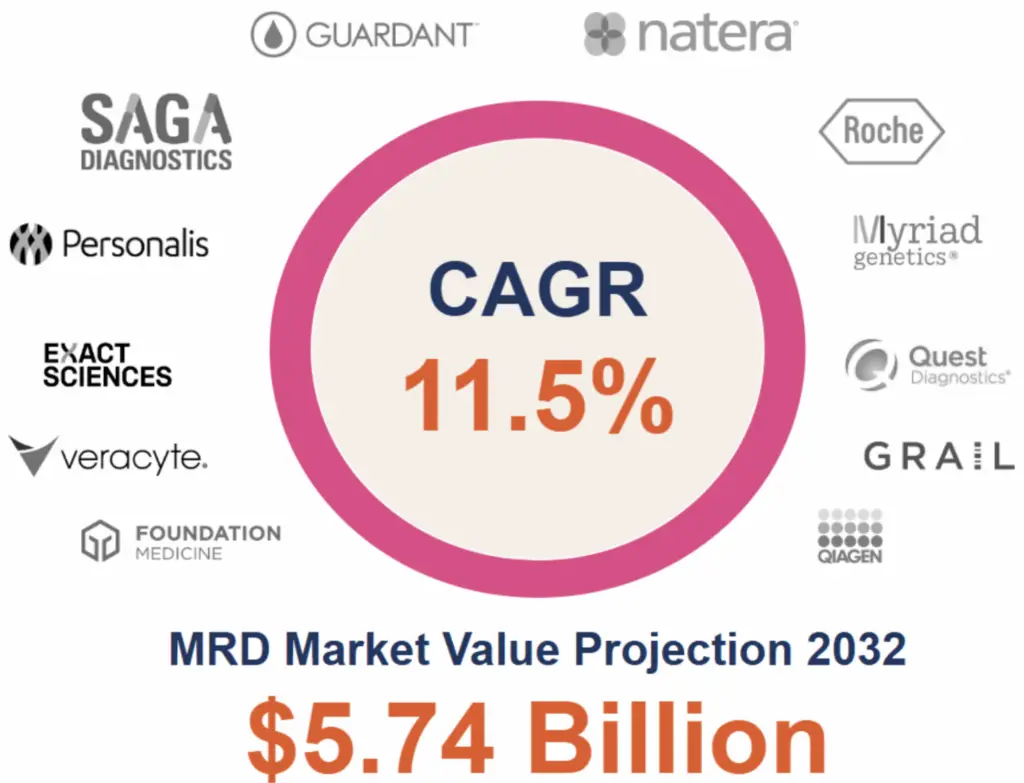
Fast forward to today, and MRD has burst onto the scene as a powerhouse in oncology. The industry is exploding, with the global MRD testing market, valued at $2.16 billion in 2023, projected to surpass $5.74 billion by 2032, which was largely driven by advances in precision oncology and the urgent need for early relapse detection.
What sparked this growth? A perfect storm of rising cancer incidence, regulatory tailwinds (including the U.S. Food and Drug Administration’s greenlighting MRD as a surrogate endpoint in multiple myeloma trials), and an influx of innovative companies. Players such as Natera, SAGA Diagnostics, Foundation Medicine, Personalis, Myriad Genetics, Guardant Health, Haystack (Quest Diagnostics) and Exact Sciences are leading the charge, offering differentiated technologies that promise to transform cancer management from reactive to proactive.
The breakthrough came with next-generation sequencing (NGS) and circulating tumor DNA (ctDNA) analysis, which catapulted MRD detection into a new era of ultra-sensitivity (down to 1 ppm or better in some instances). Companies have engineered assays that amplify single base mutations, structural variants, or epigenetic markers, to minimize false positives while maximizing detection rates.
To highlight the diversity in this space, let’s look at three distinct technological approaches used by leading MRD companies today.
One approach uses personalized, tumor-informed ctDNA analysis that starts with whole-exome sequencing of the patient’s tumor to identify unique mutations. This bespoke method enables highly sensitive monitoring of MRD post-surgery or during surveillance, which can help guide adjuvant therapy decisions and spot recurrence months ahead of imaging.
Another strategy employs a blood-based, tumor-naive method to detect ctDNA that also incorporates epigenomic analysis of methylation patterns. This tissue-free technique achieves high sensitivity that helps oncologists not only detect residual disease early for timely and personalized adjuvant therapy decision-making, but also quantifies disease burden.
And lastly, a third approach leverages stable, truncal structural variants rather than potentially spurious point mutations. By creating a personalized “genomic fingerprint” from tumor tissue, this method offers strong sensitivity across various cancer types, and supports early-stage detection and treatment response monitoring. It’s particularly effective in setting where mutations may evolve, such as metastatic disease, allowing clinicians to adapt therapeutic strategies throughout the patient’s cancer journey.
As one can see, these differentiated scientific modalities to MRD testing — ranging from tumor-informed mutations to tumor-naive epigenomic analysis and structural variant profiling — offer powerful ways to detect residual disease. This variety ensures clinicians can tailor monitoring and treatment strategies to individual patients, thereby improving early intervention and outcomes across a wide range of cancer indications.
Future Trends Pushing MRD Into Its Golden Age
As someone who’s witnessed the evolution of life sciences technologies firsthand, I believe MRD is on the cusp of its golden era. The focus has shifted from simply detecting cancer’s remnants to delivering actionable insights that integrate MRD into every stage of cancer care.
Advancements in technology and clinical practice are driving this shift. Innovations such as artificial intelligence-enhanced NGS and hybrid platforms that combine ctDNA with proteomics are pushing detection limits lower while making tests more cost-effective. These tools enable highly sensitive, patient-specific assays that support real-time monitoring through simple blood draws, which is a potential game-changer for personalized medicine across liquid and solid tumors.
While MRD testing first gained traction in hematologic malignancies, its use is expanding rapidly into solid cancers such as breast and lung, in which it’s showing strong prognostic value in adjuvant settings. Meanwhile, growing regulatory momentum, including breakthrough designations and harmonized standards, is ensuring these tests become more accessible worldwide. Together, these developments not only promise incremental gains, but also exert a profound impact that can extend survival and enhance quality of life for patients.
Closing Thoughts
The future of MRD is undeniably bright. As diagnostic companies continue to innovate, we’re not just detecting cancer’s whispers, we’re also silencing them. For patients, this means earlier interventions, tailored treatments, and a real shot at outpacing the disease. At HDMZ, we’re excited to be part of helping MRD technologies come of age, while supporting the visionaries who are moving the needle in oncology. Let’s keep pushing forward, because in the war on cancer, every residual cell counts.